Angew. Chem. Int. Ed.: Important progress has been made in the research of cathode materials for polyanionic sodium ion batteries
dentifying the Structural Evolution of the Sodium Battery Na2FePO4F Cathode material is published in the Journal of Applied Chemistry (Angew. Chem.Int. ED., 2018, 57,11918-11923).
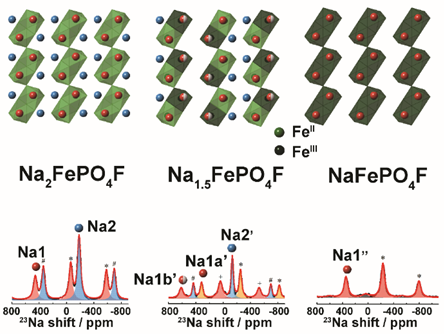
Yong Yang’s research team in the early stage of the lithium/sodium ion battery cathode material based on the reaction mechanism of solid nuclear magnetic resonance (NMR) research, combined with high in situ XRD technology (HEXRD), further study on the theoretical calculation of DFT Na2FePO4F materials in the process of charging and discharging long-range changes and ion/electronic structure, short-range LAN environment, further elaborated the electrochemical reaction mechanism of the material. In the previously reported solid state NMR results of Na2FePO4F, due to the limitations of its experimental methods, the spectral analysis and the analysis of the electrochemical reaction mechanism of the material were improper. In this study, it was found that there were two biphase reactions in the charging process of the material, namely Na2FePO4F→Na1.5FePO4F and Na1.5FePO4F→NaFePO4F. The crystal structure of the mesophase Na1.5FePO4f was obtained by DFT calculation, which was supported by the results of XRD finishing, solid state NMR experiment and paramagnetization degree shift calculation.
Professor Yang's research group has long been committed to the basic research of electrode materials for sodium ion batteries, and has made a series of important progress in the synthesis and reaction mechanism of polyanion cathode materials for different sodium ion batteries. For example, solid-state NMR technology using 23Na, 51V NMR and other nuclei combined with a variety of characterization methods proposed that the Na ions on the two Na sites could be simultaneously disembed during the initial charging stage of Na3V2(PO4)2F3. The structural evolution of Na4Fe3(PO4)2(P2O7) during the desodium process was elucidated. The reason of the poor reversibility of Na3VCr(PO4)3 multi-electron reaction is explained, and the stable reversible 1.5 electron cycle is realized at low temperature. A kind of Na3V3(PO4)4, which can be used as cathode and cathode material of sodium ion battery, was synthesized and identified. Chem. Mater. 2014, 26, 2513−2521; J. Power Sources. 2016, 327, 666-674; ACS Appl. Mater. Interfaces, 2017, 9, 43632 -- 43639; ACS Appl. Energy Mater., 2018, 1, 3603 -- 3606. In addition, based on the accumulated work of Prof. Yong Yang's group, they were invited by Small Methods to write a review paper entitled "Research Progress on Multielectron Reactions in Polyanionic Materials for sodium-ion Batteries".
The research work was completed under the guidance of Professor Yong Yang, with doctoral student Qi Li as the first author, and 2009 graduate doctor Zigeng Liu as the co-corresponding author. Professor Shunqing Wu from the School of Physical Science and Technology of Xiamen University and Professor Clare P. Grey from the University of Cambridge have provided important support in theoretical calculation. This research was supported by the Key Research and Development Program of the Ministry of Science and Technology (2016YFB0901502 and 2018YFB0905400) and the National Natural Science Foundation of China (21233004, 21761132030 and 21621091).
Paper link:https://onlinelibrary.wiley.com/doi/abs/10.1002/anie.201805555
